Background
Myelodysplastic syndrome (MDS) and Acute Myeloid Leukemia (AML) are well-known complications of traditional chemotherapy used to treat different solid and hematological malignancies. It is also reported with some newer drugs (eg. PARP inhibitors). We sought to analyze the real-world data of different chemotherapeutic drugs causing MDS/AML
Methods
From the FDA Adverse Events Report System (FAERS) public dashboard, we obtained the data on all the events reported as MDS and AML. Data was available for the years 1970 - 2023, and the events were analyzed for different drugs.
Results
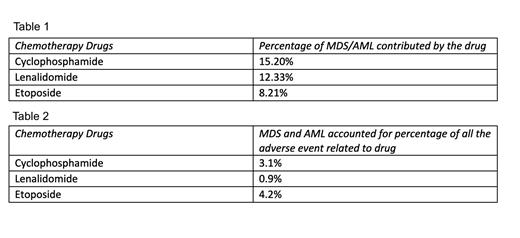
A total of 24,565 events of MDS and AML were reported to the FAERS database between 1970 and 2023. 11,045 (44.97%) occurred in males, 9,803 (39.91%) in females, and 3,714 (15.12%) events had no sex specified. Most of them were severe AEs, and 11,486 (46.7%) of them were fatal. Among all the different chemotherapeutic drugs, the highest number of reports were related to Cyclophosphamide - 3,735 events (15.20%), followed by Lenalidomide - 3,028 events (12.33%) and Etoposide - 2,017 events (8.21%) (Table 1). When individually analyzed, MDS and AML accounted for 3.1% of all AEs related to Cyclophosphamide, compared to 0.9% for Lenalidomide AEs, and 4.2% for Etoposide AEs (Table 2).
Conclusions
Based on this retrospective analysis, we found that cyclophosphamide was the most common drug associated with MDS/AML reported to the FAERS, followed by Lenalidomide and Etoposide. However, MDS/AML was a more common AE with Etoposide, than with Cyclophosphamide or Lenalidomide. Considering the fatality rates of these events, a comprehensive risk-benefit and possible alternative therapy discussion must be held with the patients prior to treatment planning.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal